A lithium-ion battery is a rechargeable battery which operates by intercalating and de-intercalating lithium ions into the anode and cathode depending on whether the electrochemical cell is being charged or discharged. Common battery chemistries currently in use today include lead-acid, nickel cadmium (NiCd), nickel metal hydride (NiMH), and lithium ion (Li-ion). Li-ion technology addressed a number of challenges associated with conventional battery chemistries. The attractive qualities of lithium-ion batteries, coupled with ongoing improvements, are contributing to the rising wave of wearable electronics, smart sensors for the Internet of Things (IoT), smartphones with high-speed processors, electric transportation, and energy harvesting from the renewable sources.
[H2]Electrochemistry[/H2]A lithium-ion battery works by migration of lithium ions through an electrolyte from the negative electrode (anode) to the positive electrode (cathode) during battery discharge, and from the positive electrode to the negative electrode as the battery is discharged. The charging process is referred to as intercalation and the discharging process is referred to as de-intercalation. Electrochemical intercalation is a mechanism of inserting guest ions into the host structure using an electric field to overcome reaction barriers—and its reverse is called electrochemical de-intercalation. Lithium ion batteries are dual intercalation systems. Both of their positive and negative electrodes have structures that allow reversible insertion and extraction of lithium ions. During discharge the anode undergoes an oxidation reaction which frees electrons to conduct current through an external circuit to the cathode which undergoes a reduction reaction (gaining of electrons). Lithium ions migrate internally through the cell via the non-aqueous electrolyte and embed themselves into the structure of anode material during the charge cycle. During the charge cycle electrons travel from the cathode through the external circuit to the anode while lithium ions leave the cathode and move across the electrolyte to the cathode.
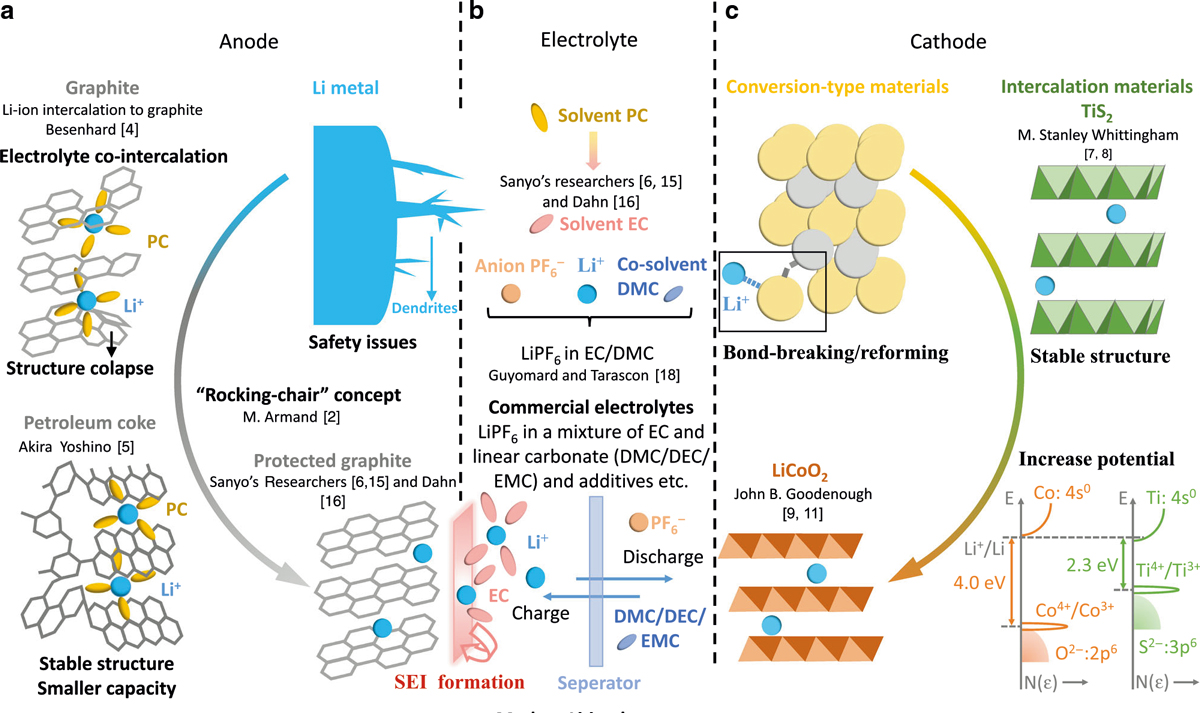
[H2]Battery Composition[/H2]A lithium-ion battery consists of a cathode and an anode typically sandwiched by a separator which provides electrical isolation between the electrodes while the electrolyte contained in the separator allows for ionic conductivity. The design and selection of cathode, anode, separator, and electrolyte largely dictate the performance characteristics of a rechargeable battery. Lithium is the lightest metal and highly reactive. The low mass gives lithium ions a greater degree of mobility than other cations while high chemical reactivity allows lithium to store large amounts of energy in its atomic bonds, thus lithium ion batteries carry a very high energy density.
The storage capacity of the lithium-ion battery is often determined collectively by the Li-ion storage capacity (represented in Ampere hour or Ah) and the discharge potential of the cell. The capability of the host, or the electrode, to change the valence states; the available space to accommodate the lithium-ions; and the reversibility of the intercalation reactions are three main factors affecting the Li-ion storage capacity through intercalation. The cathode and anode materials are critical to the performance of a LIB because they are the hosts of lithium ions in either charging or discharging cycles. The ability of the electrolyte to conduct lithium ions between the anode and the cathode and the ability of separator to isolate the electrodes from each other to prevent short circuiting are also indispensable considerations.
Since lithium ions can be intercalated into different host materials and different separator/electrolyte systems are available, "Li-ion" does not specifically correspond to a particular battery chemistry as lead acid or NiMH does. The term “lithium-ion battery” refers to a large and diverse family of different battery chemistries which may also come in different form factors, sizes, and cell constructions.
[H2]Cathode[/H2]The specific energy that a lithium ion battery is able to give is a function of the electrodes' capacity for lithium intercalation and the cell voltage. However, the cathode material is the limiting factor because it typically has a lower lithium ion storage capacity than the anode material to which it must be matched. The theoretical capacity of electrode materials corresponds to the number of reactive electrons that can be reversibly inserted and extracted as well as the molar weight of the designed materials. Lithium-ion batteries use a diversity of cathode chemistries which are mainly divided into three categories: chalcogenides, transition metals and polyanion compounds. The most commonly studied and widely used cathode materials for lithium intercalation are transition metal compounds, oxides, or complex oxides that have layered, spinel, or olivine structures. The advantages of using transition metal oxides as cathode materials are their higher operating voltage and their variable valence states that facilitate more electron-storing sites.
Lithium ion batteries are commonly classified by the composition of their positive electrodes.
Lithium cobalt oxide (LCO)
Lithium cobalt oxide (chemical formula LiCoO2) is a cathode chemistry widely used for its ability to produce cells with high volumetric energy density. However, Li-ion batteries using LCO cathodes perform poorly at low temperature performance and may have safety issues at high temperatures. LCO batteries have a cost challenge as the cathode is entirely made of cobalt. Nonetheless, LCO cathodes remain a popular chemistry for laptop, mobile phone and tablet batteries.
Lithium-iron phosphate (LFP)
Lithium-iron phosphate (LiFePO4) possesses improved thermal and chemical stability which makes it safer to use as a cathode than other lithium chemistries. The LFP chemistry has a lower risk of thermal runaway because the iron-phosphorus-oxygen bond is stronger than the cobalt-oxygen bond. LFP batteries have lower energy density than batteries using competing Li-ion chemistries; however, they have faster discharge rates, longer cycle life, and a lower cost (because the cathode is made out of more abundant iron and phosphate).
Lithium manganese oxide (LMO)
Lithium manganese oxide (LiMn2O4) provides a higher cell voltage and has better thermal stability than LCO chemistries, despite the slightly lower specific energy (100-150 Wh/kg compared to 175-240 Wh/kg) and higher capacity loss during storage or cycling. Their spinel structure leads to lower overall capacities and poor stability at higher temperatures in Li-based electrolyte. LMO is similar to LFP in performance, but is two to three times cheaper.
Lithium nickel cobalt aluminum oxide (NCA)
Lithium nickel cobalt aluminum oxide (LiNiCoAlO2) is a cathode chemistry designed to deliver high energy density while reducing cobalt usage, effectively bringing down the cost/kWh. NCA cells are considered somewhat safer and cheaper than LCO, however their cost, thermal performance and cycle life are still less attractive than other competing technologies.
Lithium nickel cobalt manganese oxide (NCM)
Lithium nickel cobalt manganese oxide cathode chemistry take advantage of the properties that nickel, manganese, and cobalt while also mitigating some of their negative cost, safety, and performance attributes. The ratios of nickel, manganese, and cobalt can be tailored to emphasize qualities that target specific applications. For high-power applications an even ratio (called NCM 1-1-1) does the best job. A formulation with higher nickel contents (5-3-2 or 6-2-2) allows the battery to provide higher energy density and simultaneously reduce dependence on cobalt. NCM is currently the cathode material of choice for EV manufacturers.
[H2]Anode[/H2]An anode is the reducing electrode that gives up its electrons to the external circuit and is oxidized in the electrochemical process. They are made from materials with very few electrons in its valence shell. Carbon-based anodes have been favored for their low cost, high energy capacity and low voltage versus lithium ions. They can be natural graphite, artificial graphite, or amorphous carbon. Graphite in its natural or artificial form is the best representative material in this group of anode materials. Being a crystallite and the most stable allotrope of carbon, graphite has perfect stacking of graphene layers in hexagonal (AB) form and in some cases rhombohedral (ABC) arrangements. Intercalation of lithium ions into the anode causes the graphene sheets to rearrange themselves on top of each other in AA arrangement and “staging” occurs. The favorable electrochemical properties make graphitic carbons a popular anode material in the frontier of commercial Li-ion cells.
A major drawback of graphite anodes is their low lithium intercalation capacity. Therefore, efforts have been made for developing anode materials with higher capacities. Not only new carbon materials such as carbon nanofibers (CNF), carbon nanotubes (CNT) and graphene are being explored, conversion reaction based materials and alloying reaction-based materials are vastly studied as an alternative to carbonaceous materials. Alloying reaction-based materials, which are metals that can be alloyed with lithium such as silicon (Si), germanium (Ge), tin (Sn) and their alloys, are most famous for their high theoretical capacity. An extremely high theoretical capacity and the lowest electrochemical potential render lithium metal anodes an ideal choice for high-voltage and high-energy batteries. Anodes based on nanostructured silicon which enable a large increase in Li+ ion storage are believe to the most promising anodes for advanced lithium ion batteries that can realistically outperform all kinds of carbon anodes.
[H2]Electrolyte[/H2]The electrolyte is designed to facilitate the transport of lithium ions from the anode to the cathode during electrochemical reactions within the cell. Typically, the transport medium is lithium salt in an organic solvent containing “lithiated” ions. The majority of electrolytes used in LIBs use lithium hexaflourophosphate (LiPF6) as the lithium salt, and ethylene carbonate (EC), dimethyl carbonate (DMC) diethyl carbonate (DEC), ethyl methyl carbonate (EMC), or propylene carbonate (PC) as the organic solvent. The combination of LiPF6 and carbonates is selected because of their high conductivity and solid electrolyte interface (SEI) forming ability. The electrolyte is typically a non-aqueous (aprotic) liquid, but can also be a gel or polymer. Polymer electrolytes are often mixed in composites with ceramic nanoparticles, which lend electrolytes higher conductivities and resistance to higher voltages. Solid electrolytes made from lithium-ion conductive crystals and ceramic glasses have been attracting significant attention but they are hardly ever used in batteries with solid electrodes due to the challenging solid−solid interfaces.
[H2]Separator[/H2]The separator in a lithium ion battery is a thin film soaked/coated with a non-aqueous electrolyte. The microporous separator can be made of polyethylene (PE), polypropylene (PP), or composite materials of PE and PP. The separator plays a central critical role as it enables lithium ion transport but prevents direct contact between the electrodes. It must be designed to prevent dendrites (metal slivers) from growing from the anode to the cathode. The internal bridge between the electrodes as a result of dendrite formation will short the circuit, followed by a thermal runaway.
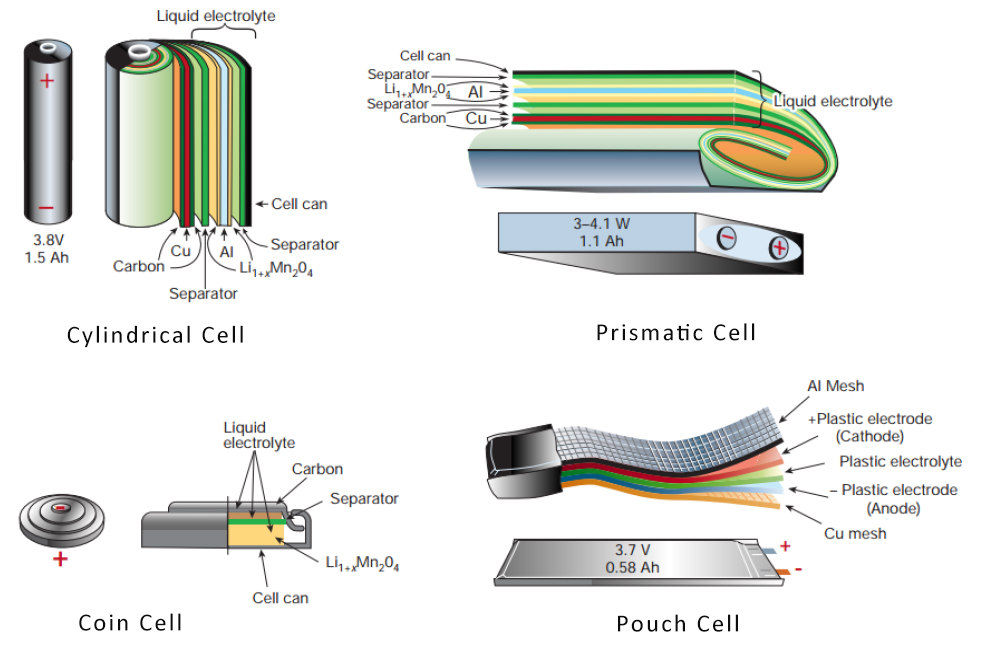
[H2]Cell Design[/H2]Lithium ion cells are available in a range of form factors. Cylindrical, prismatic, polymer/pouch, and button/coin cells are the four most commoditized forms of lithium ion cells. Cylindrical cells are made by inserting rolled strips of cathode foil, separator, and anode foil into a rigid stainless steel or aluminum cell housing. Prismatic cells are similar in construction to cylindrical cells but come in a rectangular cuboid shape for the purpose of lowering the overall thickness. Both the cylindrical and prismatic cells use a liquid electrolyte to carry charge between the anode and cathode. A built-in safety mechanism is incorporated to shut off current flow out of the battery and release internal pressure from gas buildup in case of a cell abuse event. Pouch cells are cells where rectangular stacks of individual electrode/separator layers are contained within a soft plastic-aluminum package. They save on cost, weight, and thickness by eliminating the use of a rigid metal case, but may not offer the same safety and durability qualities of cylindrical and prismatic cells. Button cells are shaped like a coin or a button for use in small devices such as cameras, hearing aids, calculators, and electronic watches.
[H2]Advantages[/H2]While many chemistries are available in the market today, lithium ion batteries deliver fundamental advantages over other chemistries. Lithium ion systems are by far the best-performing rechargeable systems in terms of energy density, specific energy, charge efficiency, charge retention, cycle life, and depth of discharge (DoD). Lithium is a highly reactive element with a high electropositivity, very small radius, and very low reduction potential. These characteristics combined with the discovery of different inorganic compounds that react with alkali metals in a reversible way have opened doors to the design of rechargeable batteries with a light weight, small size, high capacity, low memory effect, long life span, high and stable voltage profile. Rechargeable lithium ion batteries have an energy density of up to 600+ Wh/Kg, a specific energy approaching 300 Wh/Kg, a power density of up to 5000 W/kg, a 80 percent DOD, a cycle life of up to 3000 discharges, and a self-discharge rate or internal electrochemical “leakage” of between 2% and 3% per month.
[H2]Disadvantages[/H2]These fundamental advantages of lithium ion batteries, however, are slightly overshadowed by their disadvantages. These batteries suffers from significant safety concerns related to battery cell abuse and improper operation. Overcharging, overdischarging, short circuiting, physical damage to the battery, or operating above the maximum operating temperature can result in thermal runaway (an irreversible meltdown). Thermal runaway often results in fire and/or explosion. As over-charging of a lithium-ion cell, high ambient temperatures, internal heating of the cell during use and internally generated heat from a short-circuit cell failure pose serious damage potentials, additional protective circuitry is required to monitor and control battery operation. A battery management system (BMS) would manage battery voltage, current, charge and discharge rate, temperature and pressure, and may include a mechanism for optimizing cycle life.
[H2]Applications[/H2]Since it was first commercially introduced by Sony Corporation on 1991 using lithium cobalt oxide (LiCoO2) as the cathode material and mesocarbon microbeads as the anode material, the lithium-ion battery has become the battery of choice for a wide variety of applications.
[H2]Electrochemistry[/H2]A lithium-ion battery works by migration of lithium ions through an electrolyte from the negative electrode (anode) to the positive electrode (cathode) during battery discharge, and from the positive electrode to the negative electrode as the battery is discharged. The charging process is referred to as intercalation and the discharging process is referred to as de-intercalation. Electrochemical intercalation is a mechanism of inserting guest ions into the host structure using an electric field to overcome reaction barriers—and its reverse is called electrochemical de-intercalation. Lithium ion batteries are dual intercalation systems. Both of their positive and negative electrodes have structures that allow reversible insertion and extraction of lithium ions. During discharge the anode undergoes an oxidation reaction which frees electrons to conduct current through an external circuit to the cathode which undergoes a reduction reaction (gaining of electrons). Lithium ions migrate internally through the cell via the non-aqueous electrolyte and embed themselves into the structure of anode material during the charge cycle. During the charge cycle electrons travel from the cathode through the external circuit to the anode while lithium ions leave the cathode and move across the electrolyte to the cathode.
[H2]Battery Composition[/H2]A lithium-ion battery consists of a cathode and an anode typically sandwiched by a separator which provides electrical isolation between the electrodes while the electrolyte contained in the separator allows for ionic conductivity. The design and selection of cathode, anode, separator, and electrolyte largely dictate the performance characteristics of a rechargeable battery. Lithium is the lightest metal and highly reactive. The low mass gives lithium ions a greater degree of mobility than other cations while high chemical reactivity allows lithium to store large amounts of energy in its atomic bonds, thus lithium ion batteries carry a very high energy density.
The storage capacity of the lithium-ion battery is often determined collectively by the Li-ion storage capacity (represented in Ampere hour or Ah) and the discharge potential of the cell. The capability of the host, or the electrode, to change the valence states; the available space to accommodate the lithium-ions; and the reversibility of the intercalation reactions are three main factors affecting the Li-ion storage capacity through intercalation. The cathode and anode materials are critical to the performance of a LIB because they are the hosts of lithium ions in either charging or discharging cycles. The ability of the electrolyte to conduct lithium ions between the anode and the cathode and the ability of separator to isolate the electrodes from each other to prevent short circuiting are also indispensable considerations.
Since lithium ions can be intercalated into different host materials and different separator/electrolyte systems are available, "Li-ion" does not specifically correspond to a particular battery chemistry as lead acid or NiMH does. The term “lithium-ion battery” refers to a large and diverse family of different battery chemistries which may also come in different form factors, sizes, and cell constructions.
[H2]Cathode[/H2]The specific energy that a lithium ion battery is able to give is a function of the electrodes' capacity for lithium intercalation and the cell voltage. However, the cathode material is the limiting factor because it typically has a lower lithium ion storage capacity than the anode material to which it must be matched. The theoretical capacity of electrode materials corresponds to the number of reactive electrons that can be reversibly inserted and extracted as well as the molar weight of the designed materials. Lithium-ion batteries use a diversity of cathode chemistries which are mainly divided into three categories: chalcogenides, transition metals and polyanion compounds. The most commonly studied and widely used cathode materials for lithium intercalation are transition metal compounds, oxides, or complex oxides that have layered, spinel, or olivine structures. The advantages of using transition metal oxides as cathode materials are their higher operating voltage and their variable valence states that facilitate more electron-storing sites.
Lithium ion batteries are commonly classified by the composition of their positive electrodes.
Lithium cobalt oxide (LCO)
Lithium cobalt oxide (chemical formula LiCoO2) is a cathode chemistry widely used for its ability to produce cells with high volumetric energy density. However, Li-ion batteries using LCO cathodes perform poorly at low temperature performance and may have safety issues at high temperatures. LCO batteries have a cost challenge as the cathode is entirely made of cobalt. Nonetheless, LCO cathodes remain a popular chemistry for laptop, mobile phone and tablet batteries.
Lithium-iron phosphate (LFP)
Lithium-iron phosphate (LiFePO4) possesses improved thermal and chemical stability which makes it safer to use as a cathode than other lithium chemistries. The LFP chemistry has a lower risk of thermal runaway because the iron-phosphorus-oxygen bond is stronger than the cobalt-oxygen bond. LFP batteries have lower energy density than batteries using competing Li-ion chemistries; however, they have faster discharge rates, longer cycle life, and a lower cost (because the cathode is made out of more abundant iron and phosphate).
Lithium manganese oxide (LMO)
Lithium manganese oxide (LiMn2O4) provides a higher cell voltage and has better thermal stability than LCO chemistries, despite the slightly lower specific energy (100-150 Wh/kg compared to 175-240 Wh/kg) and higher capacity loss during storage or cycling. Their spinel structure leads to lower overall capacities and poor stability at higher temperatures in Li-based electrolyte. LMO is similar to LFP in performance, but is two to three times cheaper.
Lithium nickel cobalt aluminum oxide (NCA)
Lithium nickel cobalt aluminum oxide (LiNiCoAlO2) is a cathode chemistry designed to deliver high energy density while reducing cobalt usage, effectively bringing down the cost/kWh. NCA cells are considered somewhat safer and cheaper than LCO, however their cost, thermal performance and cycle life are still less attractive than other competing technologies.
Lithium nickel cobalt manganese oxide (NCM)
Lithium nickel cobalt manganese oxide cathode chemistry take advantage of the properties that nickel, manganese, and cobalt while also mitigating some of their negative cost, safety, and performance attributes. The ratios of nickel, manganese, and cobalt can be tailored to emphasize qualities that target specific applications. For high-power applications an even ratio (called NCM 1-1-1) does the best job. A formulation with higher nickel contents (5-3-2 or 6-2-2) allows the battery to provide higher energy density and simultaneously reduce dependence on cobalt. NCM is currently the cathode material of choice for EV manufacturers.
[H2]Anode[/H2]An anode is the reducing electrode that gives up its electrons to the external circuit and is oxidized in the electrochemical process. They are made from materials with very few electrons in its valence shell. Carbon-based anodes have been favored for their low cost, high energy capacity and low voltage versus lithium ions. They can be natural graphite, artificial graphite, or amorphous carbon. Graphite in its natural or artificial form is the best representative material in this group of anode materials. Being a crystallite and the most stable allotrope of carbon, graphite has perfect stacking of graphene layers in hexagonal (AB) form and in some cases rhombohedral (ABC) arrangements. Intercalation of lithium ions into the anode causes the graphene sheets to rearrange themselves on top of each other in AA arrangement and “staging” occurs. The favorable electrochemical properties make graphitic carbons a popular anode material in the frontier of commercial Li-ion cells.
A major drawback of graphite anodes is their low lithium intercalation capacity. Therefore, efforts have been made for developing anode materials with higher capacities. Not only new carbon materials such as carbon nanofibers (CNF), carbon nanotubes (CNT) and graphene are being explored, conversion reaction based materials and alloying reaction-based materials are vastly studied as an alternative to carbonaceous materials. Alloying reaction-based materials, which are metals that can be alloyed with lithium such as silicon (Si), germanium (Ge), tin (Sn) and their alloys, are most famous for their high theoretical capacity. An extremely high theoretical capacity and the lowest electrochemical potential render lithium metal anodes an ideal choice for high-voltage and high-energy batteries. Anodes based on nanostructured silicon which enable a large increase in Li+ ion storage are believe to the most promising anodes for advanced lithium ion batteries that can realistically outperform all kinds of carbon anodes.
[H2]Electrolyte[/H2]The electrolyte is designed to facilitate the transport of lithium ions from the anode to the cathode during electrochemical reactions within the cell. Typically, the transport medium is lithium salt in an organic solvent containing “lithiated” ions. The majority of electrolytes used in LIBs use lithium hexaflourophosphate (LiPF6) as the lithium salt, and ethylene carbonate (EC), dimethyl carbonate (DMC) diethyl carbonate (DEC), ethyl methyl carbonate (EMC), or propylene carbonate (PC) as the organic solvent. The combination of LiPF6 and carbonates is selected because of their high conductivity and solid electrolyte interface (SEI) forming ability. The electrolyte is typically a non-aqueous (aprotic) liquid, but can also be a gel or polymer. Polymer electrolytes are often mixed in composites with ceramic nanoparticles, which lend electrolytes higher conductivities and resistance to higher voltages. Solid electrolytes made from lithium-ion conductive crystals and ceramic glasses have been attracting significant attention but they are hardly ever used in batteries with solid electrodes due to the challenging solid−solid interfaces.
[H2]Separator[/H2]The separator in a lithium ion battery is a thin film soaked/coated with a non-aqueous electrolyte. The microporous separator can be made of polyethylene (PE), polypropylene (PP), or composite materials of PE and PP. The separator plays a central critical role as it enables lithium ion transport but prevents direct contact between the electrodes. It must be designed to prevent dendrites (metal slivers) from growing from the anode to the cathode. The internal bridge between the electrodes as a result of dendrite formation will short the circuit, followed by a thermal runaway.
[H2]Cell Design[/H2]Lithium ion cells are available in a range of form factors. Cylindrical, prismatic, polymer/pouch, and button/coin cells are the four most commoditized forms of lithium ion cells. Cylindrical cells are made by inserting rolled strips of cathode foil, separator, and anode foil into a rigid stainless steel or aluminum cell housing. Prismatic cells are similar in construction to cylindrical cells but come in a rectangular cuboid shape for the purpose of lowering the overall thickness. Both the cylindrical and prismatic cells use a liquid electrolyte to carry charge between the anode and cathode. A built-in safety mechanism is incorporated to shut off current flow out of the battery and release internal pressure from gas buildup in case of a cell abuse event. Pouch cells are cells where rectangular stacks of individual electrode/separator layers are contained within a soft plastic-aluminum package. They save on cost, weight, and thickness by eliminating the use of a rigid metal case, but may not offer the same safety and durability qualities of cylindrical and prismatic cells. Button cells are shaped like a coin or a button for use in small devices such as cameras, hearing aids, calculators, and electronic watches.
[H2]Advantages[/H2]While many chemistries are available in the market today, lithium ion batteries deliver fundamental advantages over other chemistries. Lithium ion systems are by far the best-performing rechargeable systems in terms of energy density, specific energy, charge efficiency, charge retention, cycle life, and depth of discharge (DoD). Lithium is a highly reactive element with a high electropositivity, very small radius, and very low reduction potential. These characteristics combined with the discovery of different inorganic compounds that react with alkali metals in a reversible way have opened doors to the design of rechargeable batteries with a light weight, small size, high capacity, low memory effect, long life span, high and stable voltage profile. Rechargeable lithium ion batteries have an energy density of up to 600+ Wh/Kg, a specific energy approaching 300 Wh/Kg, a power density of up to 5000 W/kg, a 80 percent DOD, a cycle life of up to 3000 discharges, and a self-discharge rate or internal electrochemical “leakage” of between 2% and 3% per month.
[H2]Disadvantages[/H2]These fundamental advantages of lithium ion batteries, however, are slightly overshadowed by their disadvantages. These batteries suffers from significant safety concerns related to battery cell abuse and improper operation. Overcharging, overdischarging, short circuiting, physical damage to the battery, or operating above the maximum operating temperature can result in thermal runaway (an irreversible meltdown). Thermal runaway often results in fire and/or explosion. As over-charging of a lithium-ion cell, high ambient temperatures, internal heating of the cell during use and internally generated heat from a short-circuit cell failure pose serious damage potentials, additional protective circuitry is required to monitor and control battery operation. A battery management system (BMS) would manage battery voltage, current, charge and discharge rate, temperature and pressure, and may include a mechanism for optimizing cycle life.
[H2]Applications[/H2]Since it was first commercially introduced by Sony Corporation on 1991 using lithium cobalt oxide (LiCoO2) as the cathode material and mesocarbon microbeads as the anode material, the lithium-ion battery has become the battery of choice for a wide variety of applications.
- Li-ion chemistry have been dominating the rechargeable energy storage solutions for powering consumer electrical and electronic devices, including mobile phones, digital cameras, tablet and laptop computers, electric shavers electric toothbrushes, and headphones.
- Automotive applications which have overtaken portable devices as the biggest source of LIB demand. Li-ion batteries are the workhorse in electric vehicle (EVs), hybrid electric vehicles (HEVs), plug-in hybrid electric vehicle (PHEVs), and light-electric vehicles.
- The Li-ion battery is currently finding widespread application in industrial equipment, including cordless power tools, telecommunications systems, wireless security systems, and outdoor portable electronic equipment.
- The ever-increasing deployment of clean and renewable energy sources (e.g., sun and wind), and large-scale grid/utility storage have triggered a rapid increase in demand for lithium-ion batteries.
- These rechargeable batteries are well suited for use in LED lighting systems including solar lights, flashlights, headlamps, dive lights, outdoor security lights.
- Medical devices, including patient monitors, handheld surgical tools and portable diagnostic equipment, are also commonly powered by lithium-ion batteries.



