A valve regulated lead acid (VRLA) battery is a sealed lead acid (SLA) battery that has the electrolyte immobilized within the cell. Batteries of this type have a safety vent (pressure relief valve) to release gas in case of excessive internal pressure buildup. The VRLA battery thus gets its name from a characteristic valve built into the sealed container. VRLA batteries are designed to eliminate the inconveniences associated with using flooded or vented lead acid batteries. Conventional wet batteries must be installed in upright orientation to prevent electrolyte leakage or spillage, operate in ventilated environment to diffuse gases created during cycling, and require routine maintenance of electrolyte. By using a sealed design and an immobilized electrolyte, VRLA batteries can operate in any orientation and require no periodic topping up of the electrolyte.
[H2]Electrochemistry[/H2]VRLA batteries have essentially the same chemistry as flooded lead acid batteries. As the battery discharges, lead (Pb) at the negative plate (anode) reacts with sulfate ions in the electrolyte to produce lead sulfate (PbSO4) and electrons (e⁻). The electrons move to the lead dioxide (PbO2) positive plate (cathode) through the external electric circuit and release oxygen molecules from the lead dioxide. The presence of oxygen causes hydrogen to break its bond with the sulfate in the electrolyte and combine with oxygen to create water (H2O). The debonded sulfate molecules combine with the available lead molecules to produce lead sulfate. The cycle is reversed when the battery is in a charged state. An excess of electrons created by an external electrical charging source causes the lead molecules to release the sulfate molecules, which converts the positive and negative plate to its original form of lead dioxide and lead, respectively, and restores the sulfuric acid of the electrolyte.
[H2]Recombinant Technology[/H2]While recharging of the lead acid battery restores the potential energy, oxygen and hydrogen is created at the positive and negative electrodes as a result of the electrolysis of the electrolyte during charging, respectively. In flooded batteries, both oxygen and hydrogen percolate up through the electrolyte as gas bubbles because of the low solubility of both gases in the electrolyte. These batteries must be vented to prevent buildup of internal pressure. Also, the space or enclosure housing the flooded battery must be well ventilated since a concentrated mixture of hydrogen and oxygen poses an explosion hazard. Regular maintenance (addition of water) is required to compensate for the water loss because of water decomposition during charging.
A special feature of VRLA rechargeable batteries is that these batteries use a recombination reaction to prevent the escape of hydrogen and oxygen gases normally lost in a vented battery (particularly in deep cycle applications). The oxygen produced on the positive plate is diffused through the separator system to the negative plate. The charged negative plate reacts first with this oxygen and subsequently with the electrolyte to produce water. The negative electrode is partially discharged during charging. The partial discharge of the negative electrode is deliberated to suppress the hydrogen formation. Since very little oxygen and hydrogen will released from the battery and the water content of the electrolyte thus remains unchanged, no electrolyte maintenance is required and the battery can be sealed. The effectiveness of recombination reactions is 98 - 98 %. The excessive gas pressure buildup inside the battery caused by remaining 1 – 2 % oxygen, which can lead to the formation of hydrogen at the negative plate, is regulated by a one-way, pressure-relief valve system. If the VRLA battery is overcharged to such an extent that oxygen is generated at a rate faster than what it can be absorbed by the negative plate, gas will be vented from the valves.
[H2]Electrolyte Immobilization[/H2]VRLA batteries are acid-starved to immobilize the electrolyte and to create a porous medium. With an immobilized electrolyte, the battery can be operated in any orientation. The porous structure allows oxygen to migrate to the negative electrode without rising directly after leaving the positive electrode as gas bubbles. The “acid-starved” condition of electrolyte also helps limit the discharge the plates can deliver and protects the plates from shedding due to deep discharge. The electrolyte is immobilized either by using a sulphuric acid electrolyte soaked in a porous AGM (absorbed glass mat) separator, or by forming a gel through the addition of silicon dioxide. Hence VRLA or sealed lead acid batteries can be divided into two categories based on the mechanism of immobilization: AGM and gel batteries.
[H2]AGM Battery[/H2]An AGM battery has its electrolyte completely absorbed in separators consisting of matted glass fibers next to the plates. The electrolyte is suspended in close proximity with the electrode plates' active material. The separator has a critical role in battery performance as it holds all the required sulfuric acid to prevent uneven saturation and acid stratification, provides force to the active material to minimize premature capacity loss, and regulates the oxygen transfer from the positive to the negative plate.
AGM batteries have higher specific power, power density, discharge and recharge efficiency than flooded and gel lead acid batteries since they have very low internal resistance. The low internal resistance also contributes to a very slow self-discharge rate and a good immunity to sulfation in storage. The ability to deliver high currents on demand makes more suited to automotive SLI (Starter, Lighting and Ignition) applications than gel lead-acid batteries. Acid suspension makes the battery spill-proof which enables shipment without hazardous material restrictions. High vibration resistance due to sandwich construction suggests that AGM batteries are well-equipped to handle harsh operating environments.
AGM lead-acid batteries, however, do not tolerate overcharging and thus require the use of a charger that must specifically have an AGM setting. AGM batteries are at a significantly elevated risk of thermal runaway when the battery's float voltage is kept elevated or the rate of heat production in a battery exceeds its heat dissipation capability due to overcharging.
[H2]Gel Battery[/H2]A gel battery has its electrolyte permanently locked in a highly viscous gelled state. The oxygen transfer in gel batteries takes place through micro cracks formed in the gelled electrolyte. The gel battery are more acid-starved than AGM batteries, which give more protection to the plates. Therefore, these batteries have a superior deep discharge resiliency and outlast AGM batteries in terms of cycle life. In comparison to AGM batteries gel batteries are less prone to thermal runaways because of the high electrolyte volume and therefore the very good heat transfer. The charging voltage must be carefully regulated as fast charging can damage the battery. The physical properties of the gelled electrolyte put a limit the battery's cold temperature performance.
[H2]Applications[/H2]Gas recombination technology removes the need for regular maintenance and allow lead acid batteries to be sealed. When compared with vented batteries, VRLA batteries stand out for their superior deep cycle life, long shelf life, air transportability, gassing-free operation (unless overcharged), compatibility with sensitive electronic equipment, rugged and vibration-resistant construction, and the abilities to operate in wet environments (even under 30 feet of water), extremely cold environments (will not freeze to –20°F/–30°C), and at sea with no chlorine gas in bilge (due to sulfuric acid and salt water mixing).
VRLA batteries can be substituted in virtually any flooded lead-acid battery application, as well as excel in applications where conventional wet batteries cannot be used. These applications range from deep cycle, deep discharge applications (electric vehicles, portable power, personnel carriers, electronics, wheelchairs, floor scrubbers, marine & RB house power, sailboats, golf cars), standby and emergency backup applications (UPS, emergency lighting, solar power, telephone switching), to unusual and demanding applications (race cars, off-road vehicles, marine & RV starting, commercial equipment starting). AGM batteries are best suited to high current, high power applications which include engine starting, power sports, marine trolling, and other commercial deep cycle applications. The gel battery is particularly suited for super-deep discharge applications.
[H2]Further Classifications[/H2]While VRLA batteries are commonly classified by mechanism of electrolyte immobilization, they are also identified by their plate and container types. So-called “telecom” or “long duration” VRLA batteries are designed with thick plates for the purpose of discharging batteries continuously over a period of several hours. They are frequently not discharged below 1.75 volts per cell. So-called “UPS” or “high rate discharge” VRLA batteries are designed with thin plates for the purpose of discharging batteries over a period of only a few minutes. They are typically discharged to around 1.67 to 1.70 volts per cell.
VRLA batteries are available in versions with monobloc containers, modular containers, or battery cartridges. Monobloc containers are usually designed for 6-Volt (3 x 2-Volt cells) or 12-Volt (6 x 2-Volt cells) batteries that're typically found in small and medium battery back-up systems in data centers, network rooms and telecommunications environments. Modular VRLA batteries are almost always single 2-Volt cells that can be stacked one on top of another and connected in series and/or parallel. These modular cells are most common in telecommunication applications for high power, long duration back-up. Battery cartridges are multiples of VRLA batteries connected in a string, which facilitates quick installation and removal.
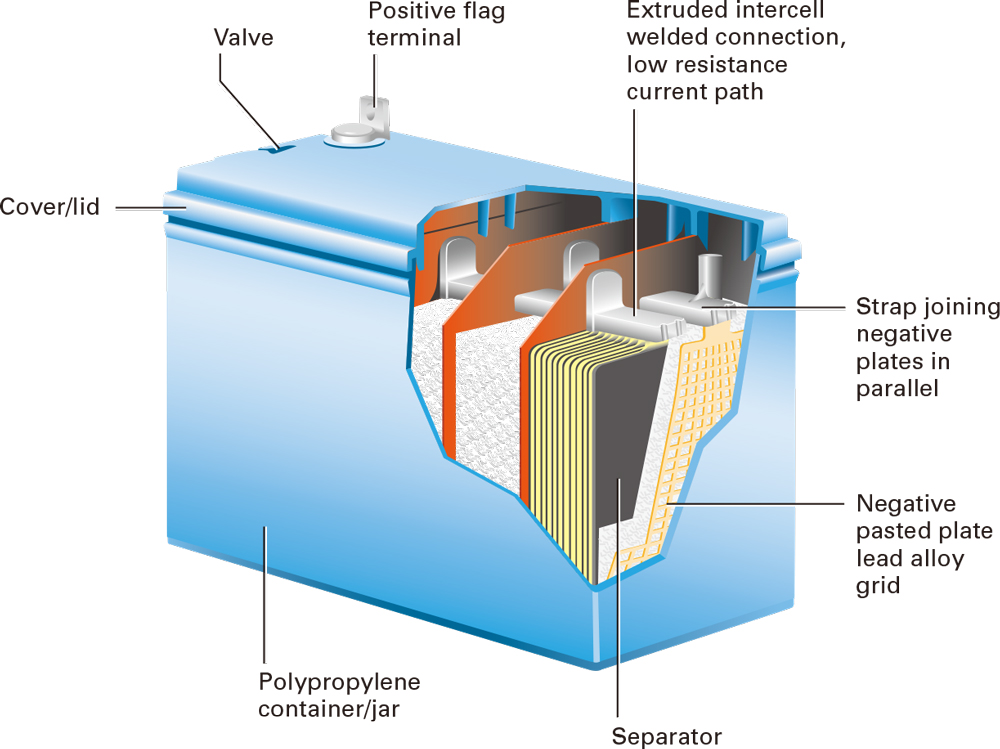
[H2]Electrochemistry[/H2]VRLA batteries have essentially the same chemistry as flooded lead acid batteries. As the battery discharges, lead (Pb) at the negative plate (anode) reacts with sulfate ions in the electrolyte to produce lead sulfate (PbSO4) and electrons (e⁻). The electrons move to the lead dioxide (PbO2) positive plate (cathode) through the external electric circuit and release oxygen molecules from the lead dioxide. The presence of oxygen causes hydrogen to break its bond with the sulfate in the electrolyte and combine with oxygen to create water (H2O). The debonded sulfate molecules combine with the available lead molecules to produce lead sulfate. The cycle is reversed when the battery is in a charged state. An excess of electrons created by an external electrical charging source causes the lead molecules to release the sulfate molecules, which converts the positive and negative plate to its original form of lead dioxide and lead, respectively, and restores the sulfuric acid of the electrolyte.
[H2]Recombinant Technology[/H2]While recharging of the lead acid battery restores the potential energy, oxygen and hydrogen is created at the positive and negative electrodes as a result of the electrolysis of the electrolyte during charging, respectively. In flooded batteries, both oxygen and hydrogen percolate up through the electrolyte as gas bubbles because of the low solubility of both gases in the electrolyte. These batteries must be vented to prevent buildup of internal pressure. Also, the space or enclosure housing the flooded battery must be well ventilated since a concentrated mixture of hydrogen and oxygen poses an explosion hazard. Regular maintenance (addition of water) is required to compensate for the water loss because of water decomposition during charging.
A special feature of VRLA rechargeable batteries is that these batteries use a recombination reaction to prevent the escape of hydrogen and oxygen gases normally lost in a vented battery (particularly in deep cycle applications). The oxygen produced on the positive plate is diffused through the separator system to the negative plate. The charged negative plate reacts first with this oxygen and subsequently with the electrolyte to produce water. The negative electrode is partially discharged during charging. The partial discharge of the negative electrode is deliberated to suppress the hydrogen formation. Since very little oxygen and hydrogen will released from the battery and the water content of the electrolyte thus remains unchanged, no electrolyte maintenance is required and the battery can be sealed. The effectiveness of recombination reactions is 98 - 98 %. The excessive gas pressure buildup inside the battery caused by remaining 1 – 2 % oxygen, which can lead to the formation of hydrogen at the negative plate, is regulated by a one-way, pressure-relief valve system. If the VRLA battery is overcharged to such an extent that oxygen is generated at a rate faster than what it can be absorbed by the negative plate, gas will be vented from the valves.
[H2]Electrolyte Immobilization[/H2]VRLA batteries are acid-starved to immobilize the electrolyte and to create a porous medium. With an immobilized electrolyte, the battery can be operated in any orientation. The porous structure allows oxygen to migrate to the negative electrode without rising directly after leaving the positive electrode as gas bubbles. The “acid-starved” condition of electrolyte also helps limit the discharge the plates can deliver and protects the plates from shedding due to deep discharge. The electrolyte is immobilized either by using a sulphuric acid electrolyte soaked in a porous AGM (absorbed glass mat) separator, or by forming a gel through the addition of silicon dioxide. Hence VRLA or sealed lead acid batteries can be divided into two categories based on the mechanism of immobilization: AGM and gel batteries.
[H2]AGM Battery[/H2]An AGM battery has its electrolyte completely absorbed in separators consisting of matted glass fibers next to the plates. The electrolyte is suspended in close proximity with the electrode plates' active material. The separator has a critical role in battery performance as it holds all the required sulfuric acid to prevent uneven saturation and acid stratification, provides force to the active material to minimize premature capacity loss, and regulates the oxygen transfer from the positive to the negative plate.
AGM batteries have higher specific power, power density, discharge and recharge efficiency than flooded and gel lead acid batteries since they have very low internal resistance. The low internal resistance also contributes to a very slow self-discharge rate and a good immunity to sulfation in storage. The ability to deliver high currents on demand makes more suited to automotive SLI (Starter, Lighting and Ignition) applications than gel lead-acid batteries. Acid suspension makes the battery spill-proof which enables shipment without hazardous material restrictions. High vibration resistance due to sandwich construction suggests that AGM batteries are well-equipped to handle harsh operating environments.
AGM lead-acid batteries, however, do not tolerate overcharging and thus require the use of a charger that must specifically have an AGM setting. AGM batteries are at a significantly elevated risk of thermal runaway when the battery's float voltage is kept elevated or the rate of heat production in a battery exceeds its heat dissipation capability due to overcharging.
[H2]Gel Battery[/H2]A gel battery has its electrolyte permanently locked in a highly viscous gelled state. The oxygen transfer in gel batteries takes place through micro cracks formed in the gelled electrolyte. The gel battery are more acid-starved than AGM batteries, which give more protection to the plates. Therefore, these batteries have a superior deep discharge resiliency and outlast AGM batteries in terms of cycle life. In comparison to AGM batteries gel batteries are less prone to thermal runaways because of the high electrolyte volume and therefore the very good heat transfer. The charging voltage must be carefully regulated as fast charging can damage the battery. The physical properties of the gelled electrolyte put a limit the battery's cold temperature performance.
[H2]Applications[/H2]Gas recombination technology removes the need for regular maintenance and allow lead acid batteries to be sealed. When compared with vented batteries, VRLA batteries stand out for their superior deep cycle life, long shelf life, air transportability, gassing-free operation (unless overcharged), compatibility with sensitive electronic equipment, rugged and vibration-resistant construction, and the abilities to operate in wet environments (even under 30 feet of water), extremely cold environments (will not freeze to –20°F/–30°C), and at sea with no chlorine gas in bilge (due to sulfuric acid and salt water mixing).
VRLA batteries can be substituted in virtually any flooded lead-acid battery application, as well as excel in applications where conventional wet batteries cannot be used. These applications range from deep cycle, deep discharge applications (electric vehicles, portable power, personnel carriers, electronics, wheelchairs, floor scrubbers, marine & RB house power, sailboats, golf cars), standby and emergency backup applications (UPS, emergency lighting, solar power, telephone switching), to unusual and demanding applications (race cars, off-road vehicles, marine & RV starting, commercial equipment starting). AGM batteries are best suited to high current, high power applications which include engine starting, power sports, marine trolling, and other commercial deep cycle applications. The gel battery is particularly suited for super-deep discharge applications.
[H2]Further Classifications[/H2]While VRLA batteries are commonly classified by mechanism of electrolyte immobilization, they are also identified by their plate and container types. So-called “telecom” or “long duration” VRLA batteries are designed with thick plates for the purpose of discharging batteries continuously over a period of several hours. They are frequently not discharged below 1.75 volts per cell. So-called “UPS” or “high rate discharge” VRLA batteries are designed with thin plates for the purpose of discharging batteries over a period of only a few minutes. They are typically discharged to around 1.67 to 1.70 volts per cell.
VRLA batteries are available in versions with monobloc containers, modular containers, or battery cartridges. Monobloc containers are usually designed for 6-Volt (3 x 2-Volt cells) or 12-Volt (6 x 2-Volt cells) batteries that're typically found in small and medium battery back-up systems in data centers, network rooms and telecommunications environments. Modular VRLA batteries are almost always single 2-Volt cells that can be stacked one on top of another and connected in series and/or parallel. These modular cells are most common in telecommunication applications for high power, long duration back-up. Battery cartridges are multiples of VRLA batteries connected in a string, which facilitates quick installation and removal.



